Simple Tips About How To Draw Sodium Atom

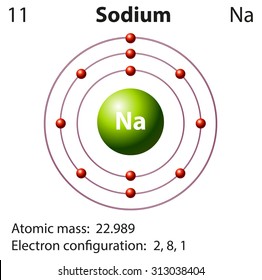
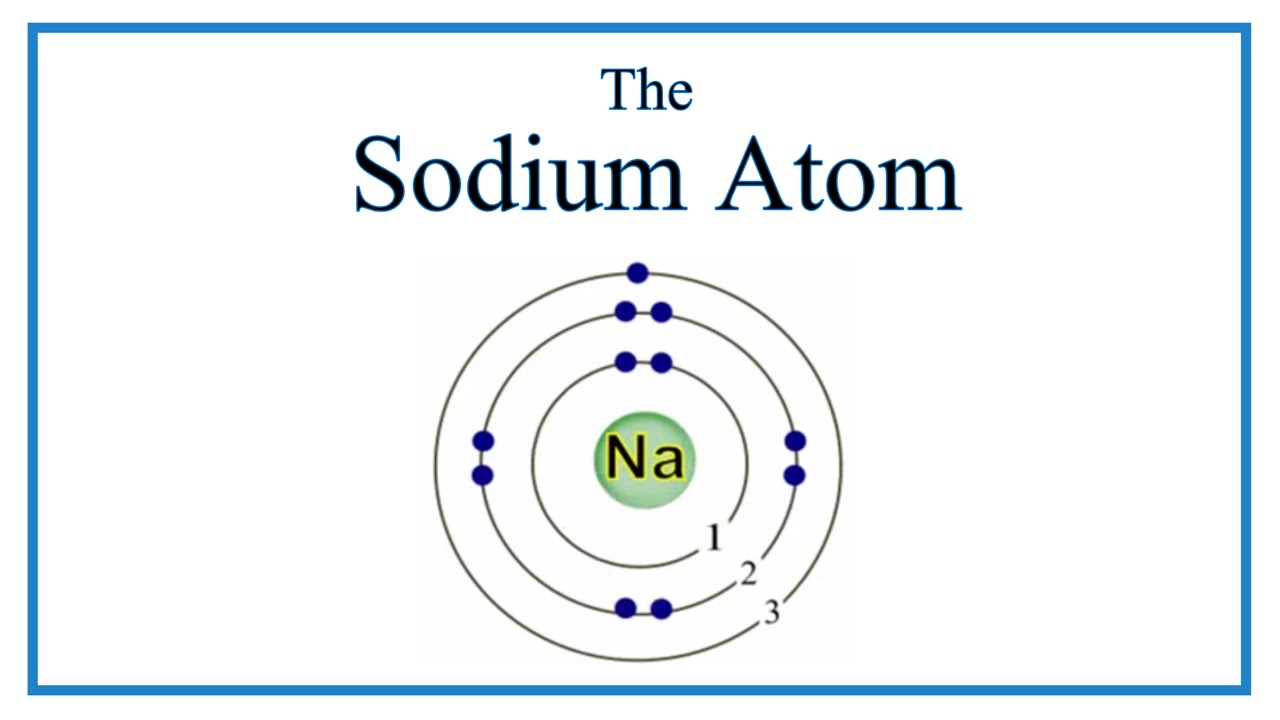
Sodium has 2 electrons in its first shell, 8 in its second and 1 in its third.check me out:
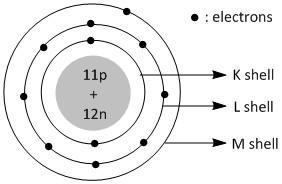
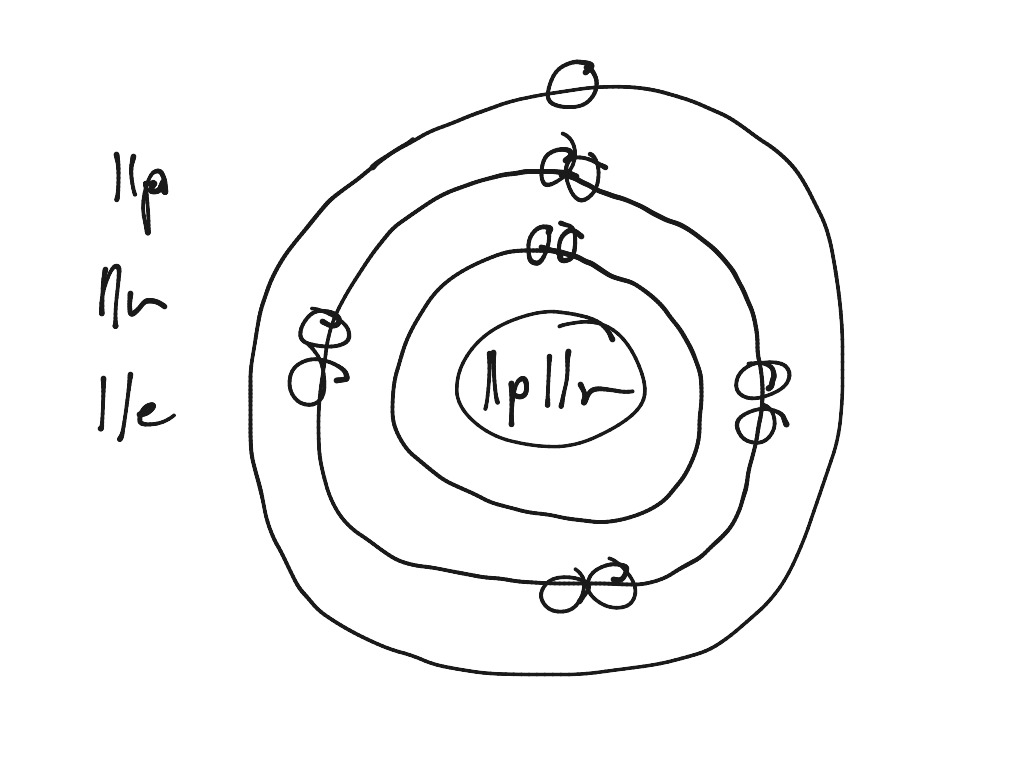
How to draw sodium atom. Draw a circle then put p for proton and n for neutron the draw a circle over the circle then you take the how many atomic number and the atomic mass and subtract the mass. We draw atomic structures for any element with the help of atomic number they have. [!]card 2 +++++[/!] [q] sodium has 11 protons, 11 neutrons, 11 electrons.
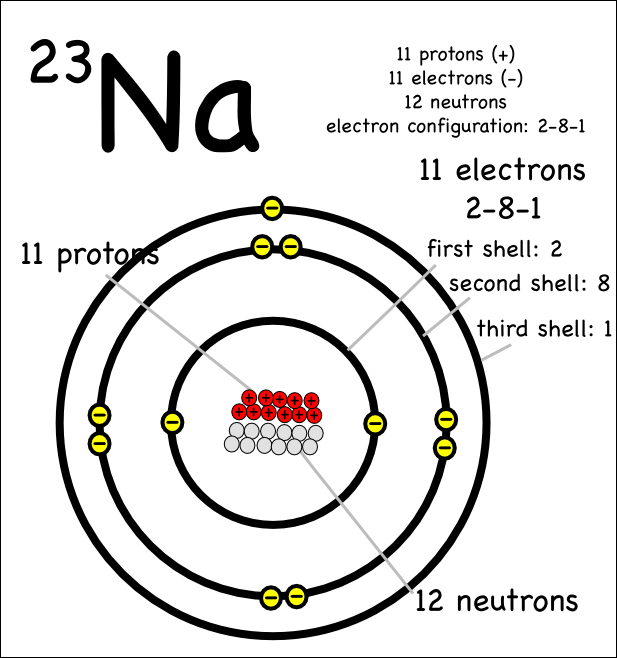
So how would you draw sodium. So, if you want to draw atomic structure for sodium first of all you should know the atomic number for. Big # rounded is 23.
Student note:this video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic table. To represent how carbon forms chemical bonds, it’s best to draw all four electrons as being unpaired. How to draw an orbital diagram for sodium?
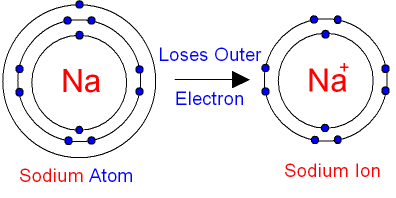
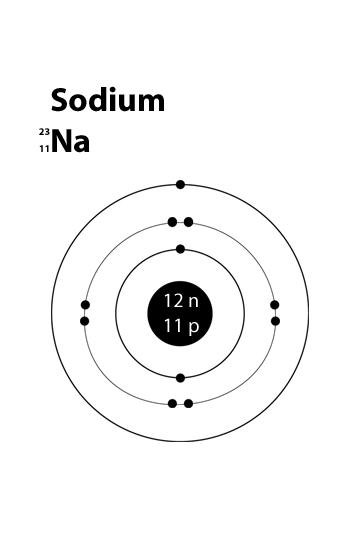
The bohr model of sodium(na) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons. Neutral sodium atom has 11 electrons whereas sodium ion (na +) has 10 electrons. Atomic mass is 35) side by side 6.
Draw a sodium atom (atomic number is 11: Atomic mass is 23 & draw a chlorine atom (atomic number is 17; Sodium has a total of 11 electrons and its electron.
In this video i have used the example of sodium, simply because it has been the first question on every chemistry paper that i have done :) Small # = 11 so protons and electrons are 11. 4 rules to drawing an atom.